Introduction: Chronic allograft enteropathy (CAE) is the main cause of intestinal graft loss two years post transplantation. Clinical course is insidious and silent, and medical treatment is disappointing. While its physiopathology is mostly unknown, the golden standard for the definitive diagnosis of CAE is histopathological analysis of the resected graft. In CAE there is atrophy of the mucosa with fibrosis and remarkable vasculopathy of middle-sized arteries. Vedolizumab, a humanised anti-α4β7 integrin antibody, is currently in use for inflammatory bowel disease (IBD). The proposed mechanism of action involves selectively blocking active immune cells from the acquired immune system from entering the intestinal mucosa. More recently, an effect on innate immune cells has also been described. In this case report, we show the first known use of vedolizumab to treat a patient with CAE.
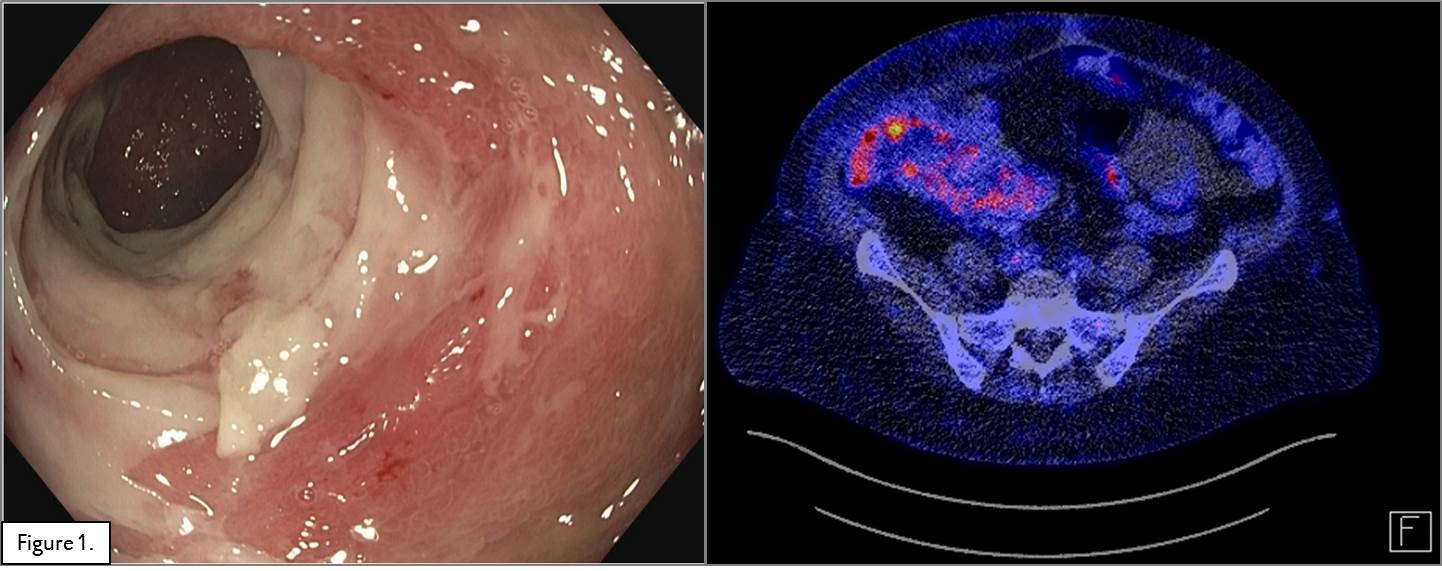
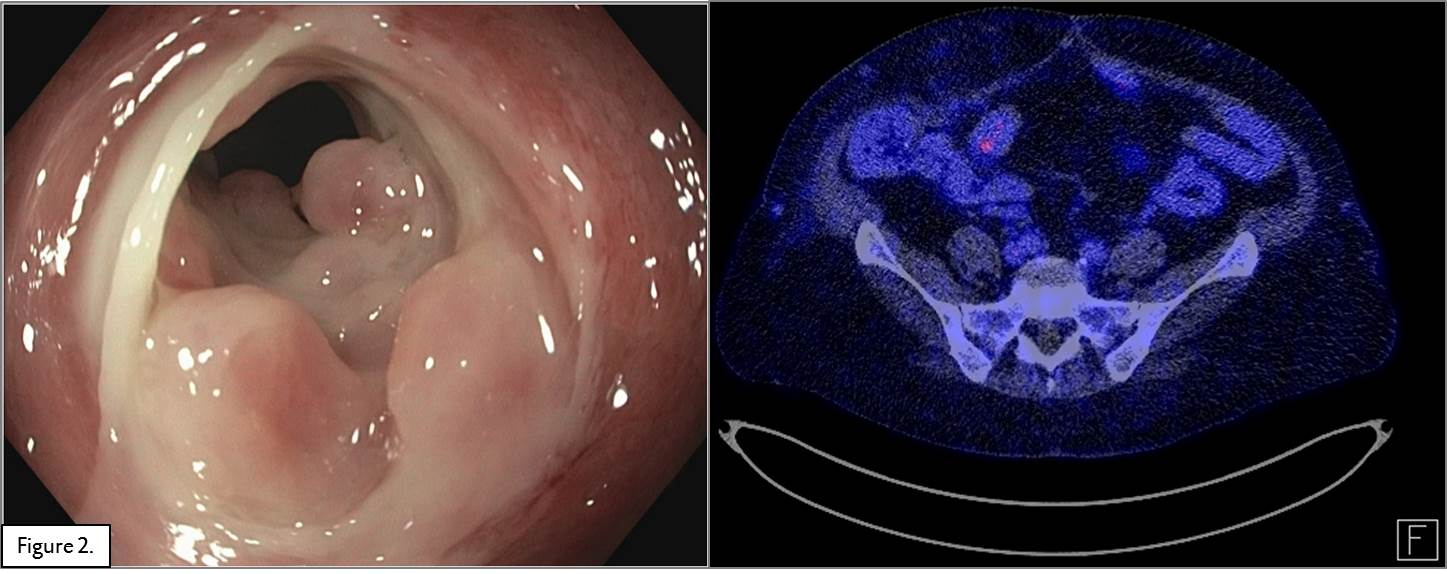
Methods and Results: A 54 year old woman,15 years post-transplant, presented with in the annual routine ileocolonoscopy ischemic ulcers and a stricture in the terminal ileum which could not be passed by the scope. A consequent PET-CT scan showed inflammatory signs in the graft (Figure 1). Mucosal biopsies showed crypt distortion and slight increase of fibrosis in the lamina propria and inflammation suggestive for CAE. HLA antibodies, PCR for EBV and CMV were all negative. Treatment with methylprednisolone (1 gram/day for 3 days) was not successful. Due to the lack of specific medical treatment for CAE, vedolizumab was started under a standard IBD scheme (300 mg I.V. at weeks 0, 2, 6 and every 8 weeks thereafter), in addition to maintenance immunosuppression. After 16 weeks (4 infusions), a follow-up colonoscopy and PET-CT scan (Figure 2) showed almost complete resolution of the mucosal inflammation, although the stricture remained. It was therefore decided to do a surgical resection of the stenotic area and continue treatment with vedolizumab.
Conclusion: This case report shows promising effects of vedolizumab in a patient with CAE. With our case study we intend to study the physiopathology of CAE to further understand how vedolizumab can play a role to extend graft survival in an otherwise deleterious condition.
.jpg )