Introduction
III-IF is a highly invalidating condition which requires long term parenteral nutrition (PN).The most frequent cause is post-surgical short bowel syndrome (SBS). Less than 50% of pts would be able to reach rehabilitation within 2 years. Favorable outcomes rely on, post-surgical intestinal length (PSIL), anatomy type (PSAT) and the presence of ICV. Over the last years the use of TED has changed the course of this disease, challenging the classically accepted predictors for favorable outcome.
Aim:
Report the experience using TED for Type III-IF in adults, single and first center experience in Latin America.
Material and Methods:
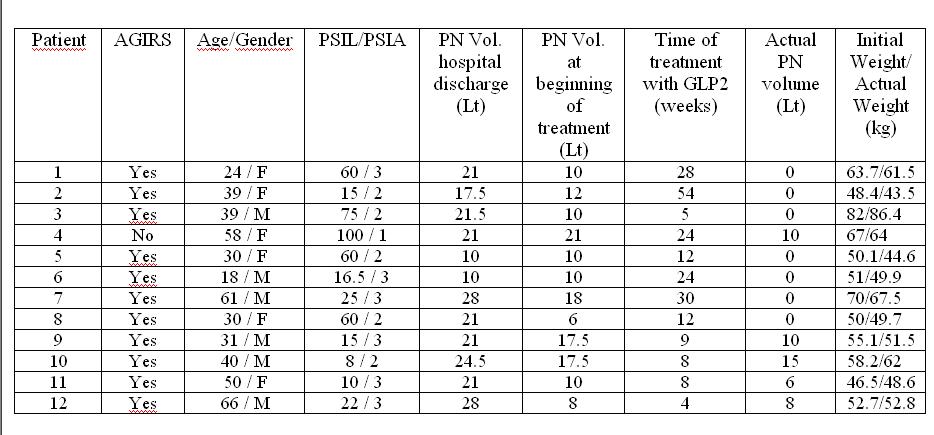
Data from TED treated pts from 2014 to 2018 was collected and analyze, including: Age, gender, PSIL: a): < 50cm., b) 51-99 cm., c)>100 cm; PSAT: 1) terminal jejunostomy, 2) jejuno-colonic anastomosis and 3) jejuno-ileo-colonic anastomosis; freedom from PN survival and comparison of outcomes to previous reports, and drug adjustments made over time, were analyzed using SPSS v20.
Results:
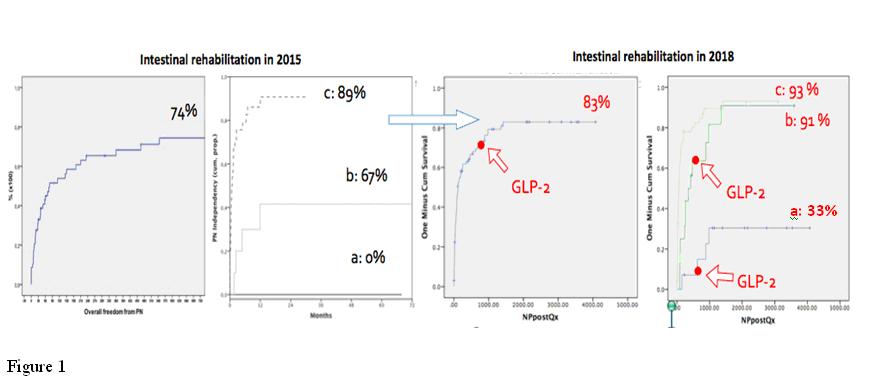
Twelve adult pts have been treated since 6/2014. 11 received AGIRS. Mean age: 40.5 years, 50% male; 7/12 had PSIL: a). Six pts had PSAT: 3); five: 2) and only one: 1). Mean PN volume during admission was 20.37 +/- 5.72 (range: 10-28) Lt/week. All patients received standard treatment for SBS, and most of them could reduce PN volume to 12.5 +/- 4.73 (range:6-21) Lt/week. 7/12 pts are currently PN free, three of them with PSIL: a) and four with PSIL:b). Compared to the 2015 report, the percentage of pts able to achieve intestinal autonomy increased from 74 % to 83%, and from those in the PSIL a) group PN independency grew from 0% to 33%, and in the PSIL b) group went from 67% to 91%. Results are shown in table (1) and figure (1). Two pts are currently off PN, and off TED; and 1 of them has been able to sustain a successful pregnancy; 2 pts have been on every other day doses due to abdominal bloating and discomfort for 14 and 7 months respectively, sustaining weight and urine output.
Conclusions:
TED is the first gut hormone commercially available proven to enhance intestinal rehabilitation in patients with SBS and III-IF. The use of TED in this very selective initial group of patients validates previous reports performed in developed countries. It adds the concept of using it after AGIRS, allowing not only to recover intestinal sufficiency but also to reduce the time to achieve it, even with unfavorably anatomy.
.jpg )