Introduction: Ischemia reperfusion injury (IRI) occurs inevitably during intestinal transplantation and after intestinal infarction. The intestine is especially susceptible to IRI which leads to loss of villi, resulting in systemic translocation contributing to poorer outcomes. The Farnesoid-X receptor (FXR), is a member of the nuclear receptor family. TGR5 is a G-protein-coupled bile activated receptor. Both are abundantly expressed in the gastro-intestinal tract. In pre-clinical models, they have shown to reduce inflammation and improve epithelial permeability when administered before ischemia. The aim of our study was to test the effect of a dual FXR/TGR5-agonist as treatment of intestinal IRI, administered intravenously after onset of ischemia.
Material and Methods: In a validated rat model (Sprague-Dawley, male, 300g) of intestinal IRI (laparotomy and clamping of superior mesenteric artery), 3 groups (n=6/group) were investigated: i/ Sham (only laparotomy); ii/ Ischemia 60min + reperfusion 60min (IRI) + intravenous vehicle; iii/ Ischemia 60min + reperfusion 60min + intravenous FXR/TGR5-agonist (IRI+FXR/TGR5). For each group, 10 additional animals were included for a 7-day survival analysis. FXR/TGR5-agonist INT-767 (Intercept Pharma, USA) or vehicle only was administered intravenously in a single dose at 10 mg/kg, 15 minutes after start of ischemia. Analyzed endpoints: 1/ Histology: Park/Chiu score and villus length; 2/ intestinal barrier function (transepithelial electrical resistance (TEER) and FD20 permeability measurements in Ussing chambers); 3/ Inflammatory cytokines: IL-6 (ELISA), IL-1-β and TNFα (qPCR); and 4/ Anti-inflammatory cytokines: IL-10, IL-13 (qPCR).
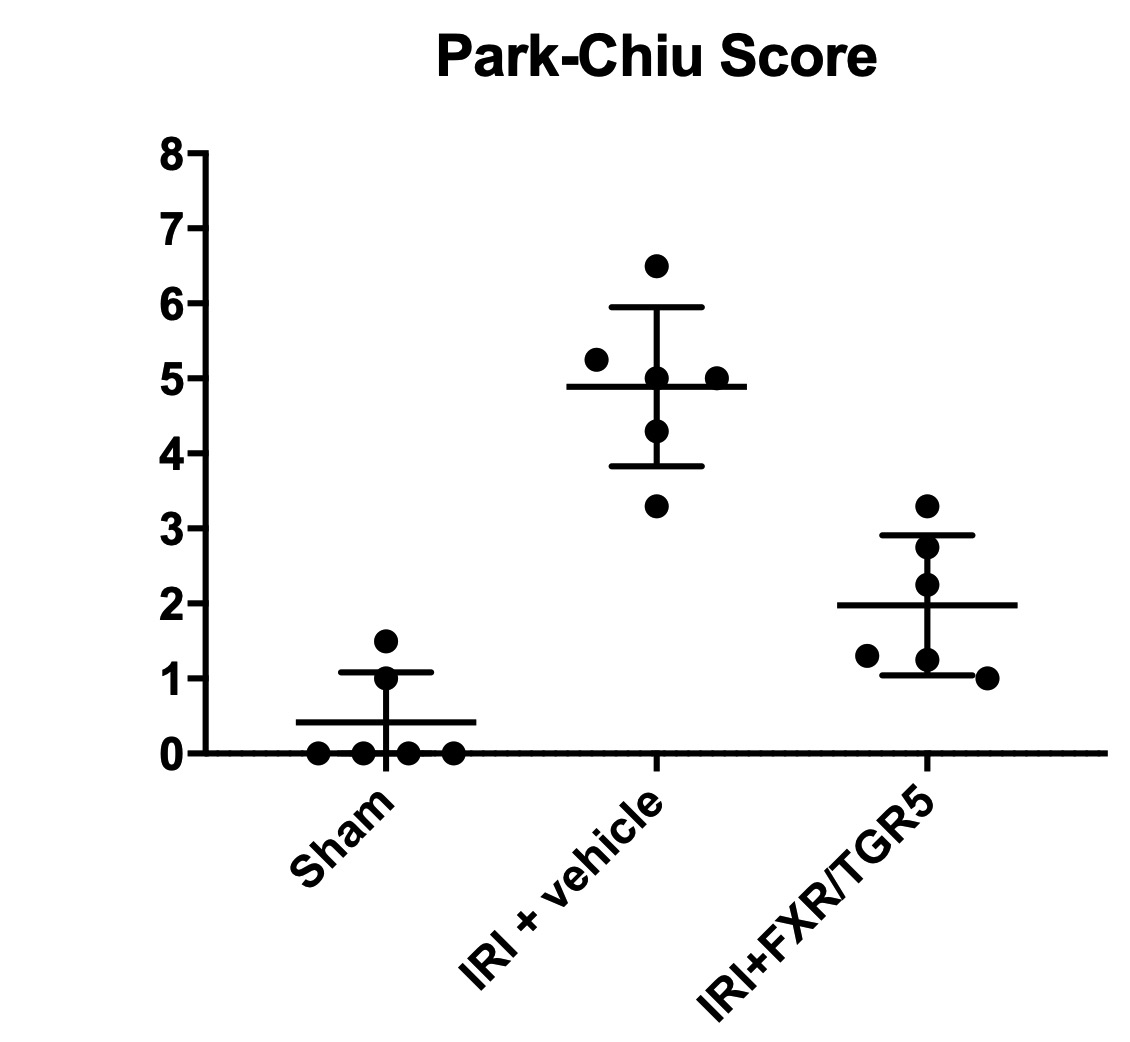
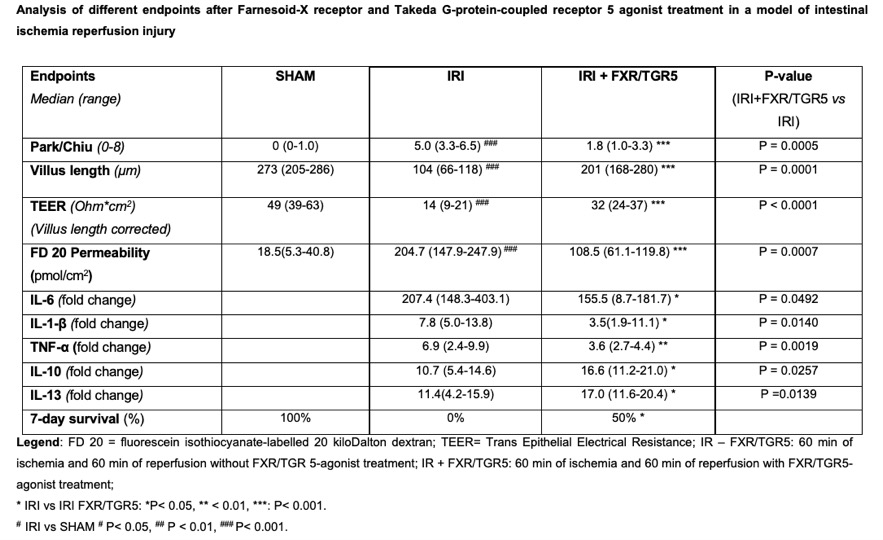
Results: IRI led to pronounced damage resulting in high Park/Chiu scores, increased intestinal permeability and systemic inflammation. Dual FXR/TGR5 treatment dramatically improved intestinal histology (Figure) and all other parameters. Survival was substantially improved after treatment (P< 0.05). Results are summarized in the table.
Conclusion: We demonstrated that intravenous treatment with a dual FXR/TGR5 agonist (INT-767) after onset of ischemia significantly decreased intestinal damage caused by IRI. These results show that FXR and TGR5 receptors are promising targets for intestinal graft protection. The ability to administer this substance intravenously greatly enhances the potential applicability for the frequent pathology of intestinal infarction as well as for transplantation.
.jpg )