Introduction
Our aim was to characterize the gut microbiota and its metabolic activity in children with IF compared with healthy controls and to explore associations with clinical parameters.
Methods
Sixty-six serial fecal samples (median of 3 per patient) were collected from 15 IF patients (median age 4.3y) dependent on parenteral nutrition (PN) for a median of 3.6y. Single samples from 25 healthy controls were collected. Gut microbiota was characterized using 16S rRNA sequencing. Short-chain fatty acids (SCFA) were quantified with gas chromatography and D and L lactate using a modified enzymatic commercial essay.
Results
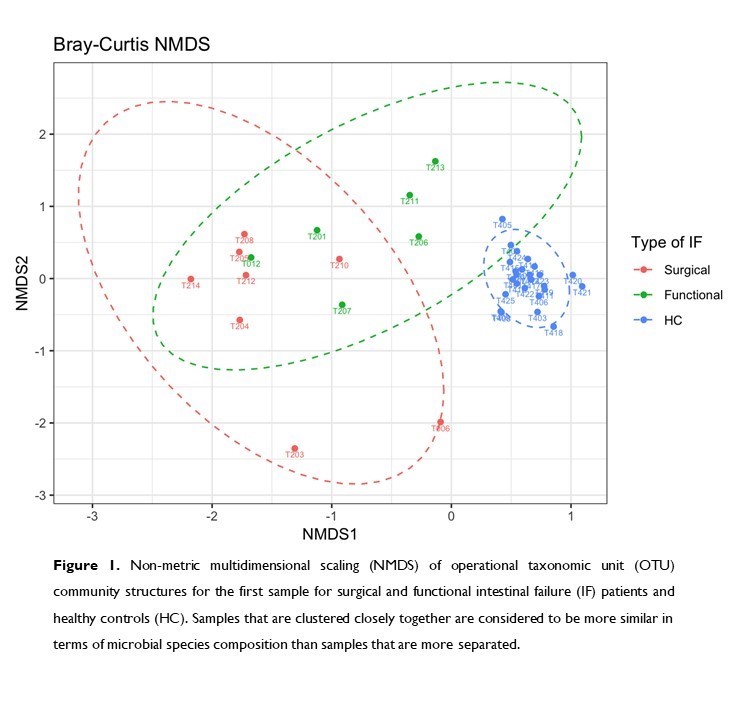
At the first sample, IF patients had lower concentration of total SCFA (p=0.008), propionic and butyric acid (p<0.001), and higher D and L lactate than controls (p<0.001). Total bacterial load (16S rRNA gene copies/g) was lower in patients (p=0.003). Their microbial community was characterized by a lower α-diversity (Shannon index, p<0.001), taxon richness (number of distinct species, Chao richness, p=0.006) and evenness (a metric of species distribution, p<0.001) than controls (Figure 1). The microbial community structure of IF patients was distinct from that of controls (p=0.002) and presented a higher degree of dispersion (inter-individual variation). Surgical IF patients had lower α-diversity (p<0.001) than functional IF patients.
Duration of PN was negatively associated with Chao richness (β=-0.29, p=0.04) and the percentage of calories provided by PN (%PN) was negatively associated with Shannon index (β=-0.33, p=0.02) and Chao richness (β=-0.34, p=0.02). Enteral fibre intake (g/kg) was positively associated with Shannon diversity (β=0.42, p<0.01). Duration of PN and %PN explained 5.5% and 6.3% of the variation in microbial community structure (p<0.01). The relative abundance of 110/200 most abundant genera was significantly different between patients and controls. Patients had higher abundance of Enterobacteriaceae, Lactobacillaceae and Staphylococcaceae, and lower abundance of Bacteroidaceae and Bifidobacteriaceae.
Conclusion
The microbiota of pediatric IF patients is distinct to that of healthy controls with altered SCFA, lower bacterial diversity, loss of dominant microbial taxa and increased abundance of sub-dominant and potentially harmful species. Associations between microbial and PN associated characteristics offer the potential to use the gut microbiota as a biomarker to guide clinical practice during intestinal adaptation.
.jpg )