Introduction: GLP2-analog treatment has been demonstrated to be efficient in adult and children patients with SBS. This study was designed to evaluate the efficacy and the safety of Teduglutide during one year in children with long-term intestinal failure due to SBS.
Methods: Children with SBS followed in our center with > 2 years on HPN, SB length < 80cm, stable on long-term PN (no decrease of PN in the past 6 months) were consecutively included in the study. At baseline they underwent a 4 days hospitalization to perform a stool balance analysis with the duplicate meal technique, blood tests, abdominal ultra-sound, densitometry, coloscopy if age >12 years old, and to initiate the treatment. Teduglutide was administered sub-cutaneously at the dose of 0.05mg/kg/day. Visits were every 2 weeks for 8 weeks, then every 4 weeks, then every 12 weeks from week 12 until week 48. At week 48, a second hospitalisation will take place to repeat the stool balance analysis. This study was registered on clinical trials NCT03562130.
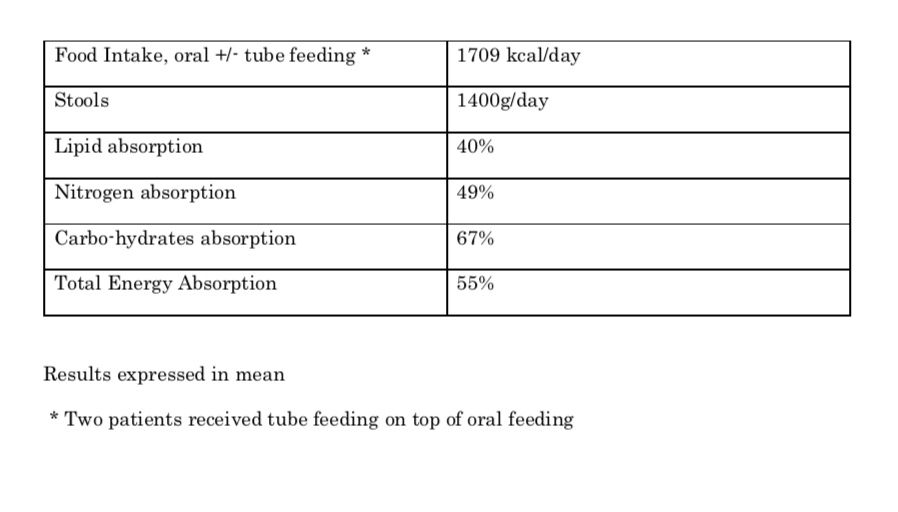
Results: Six months after the first inclusion, 12 children had been enrolled. Mean age was 10 years old (range 5-16). Two children had SBS type 1, six had type 2, and 4 type 3. The results of their stool balance analysis (baseline) are shown in table 1.
At week 12, nine children (100% children who reached the week 12 endpoint) experienced a decrease >20% of PN requirements (mean 30%). For the three children who reached week 24, further decrease of PN intake was achieved (mean 44%, from baseline).
All the children experienced a reduction in stool frequency, an improvement of the stool consistency and a reduction of the ostomy flow.
Citrulline plasma levels increased from 14 µmol/l to 26 µmol/l (mean) in the nine children who reached week 12.
Five children suffered from mild abdominal pain in the first month of treatment. Only one severe adverse event was reported with an increase ostoma output and abdominal pain which led to a hospital admission for 3 days at week 17; no direct link was made with the treatment which was maintained.
Conclusion: All the children included in the study had severe malabsorption as shown by the stool balance analysis. The first results are encouraging on the safety and the efficacy of the treatment. PN reduction still occurred after week 12 and week 24. The results of the second stool analysis at week 48 should confirm the intestinal absorption improvement that is observed clinically.
.jpg )