Introduction: Vedolizumab (VEDO) is a monoclonal antibody selectively blocking gut lymphocyte migration by blocking the α4β7 integrin. VEDO has proven its efficacy in inflammatory bowel disease (IBD). Based on the selective mode of action we tested this molecule to control chronic IBD-like inflammation.
Methods: Between September 2016 and January 2017, four patients presenting with IBD-like inflammation of the small bowel/colon resistant to conventional treatment were started on VEDO (standard induction therapy at W0-2 6 (6mg/kg) followed by maintenance therapy every 4-8 weeks). Patients were followed one year after VEDO start.
Results: All four patients had received an isolated small bowel transplant for definitive intestinal failure. All four patients had failed conventional treatment for IBD-like ileocolic chronic inflammation Median age at Vedolizumab onset was 11 years (5-20).Three patients were on triple immunosuppression with steroids, tacrolimus and azathioprine, one on steroids and tacrolimus. They received VEDO induction therapy. No adverse events were reported. Maintenance therapy was started with variable intervals and adjusted to clinical outcome. During maintenance one patient experienced a Norovirus infection and two patients had ITx rejection. The first rejection occurred after the second maintenance infusion, justifying suspension of Vedolizumab and treatment with high dose steroids, thymoglobulin and Infliximab. In the second patient rejection was treated with high dose steroids, thymoglobulin and plasmapheresis, the patient continued Vedolizumab after rejection control. Another patient stopped vedolizumab after the first maintenance infusion because of inefficacy. Two patients completed one year of treatment. Both patients stopped the treatment 15 months after its initiation because of ITx rejection. The histological analysis is ongoing to determine the immunological patterns before, during, and after VEDO treatment.
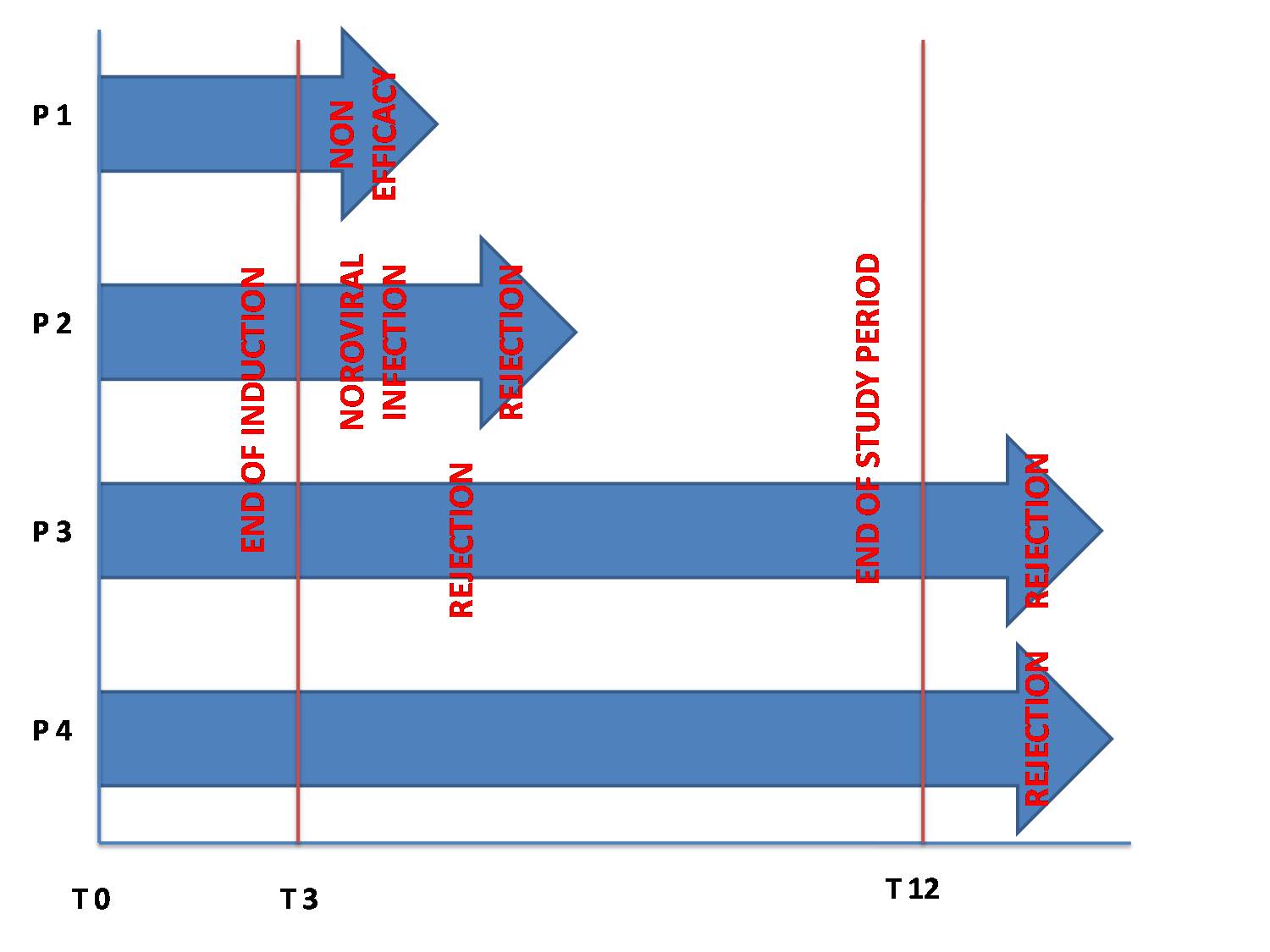
Discussion: The results of treatment for IBD-like inflammation in ITx with VEDO are disappointing. This drug do not prevent form ITx rejection relapse. Immunological pattern to elucidate possible mechanisms are currently under investigation.
.jpg )