Background: Lactoferrin (LF) is a mammalian protein produced in all bodily fluids, with its greatest concentration is in colostrum and breastmilk. LF is a known iron-binding glycoprotein and iron transporter with both antimicrobial and anti-inflammatory properties, however its exact mechanisms remain unknown. LF likely impacts the intestinal immune system, both through direct effects on immune cell development and activity, and indirect effects mediated through the gut microbiome. Studies in mice demonstrate that the gut microbiome is critical for the development and evolution of gut-associated lymphoid tissues. Segmented filamentous bacteria (SFB), members of the Firmicutes, promote proliferation of gut inflammatory Th17 cells. In contrast, other commensals stimulate Treg differentiation. We hypothesize that recombinant human LF (rhLF) ameliorates inflammation via altering the composition and function of the intestinal microbiome, promoting the development and function of Tregs.
Methods: TNFΔAREmice were administered oral vehicle (PBS), IV anti-TNF antibody (Infliximab) or oral recombinant human lactoferrin (rhLF) for 14 days by gavage. After 14 days of treatment the mice were euthanized and intestinal tissues were isolated for evaluation by Flow cytometry, ELISA, and PCR.Cecal stool samples were collected and bacterial RNA was isolatedand sequenced.
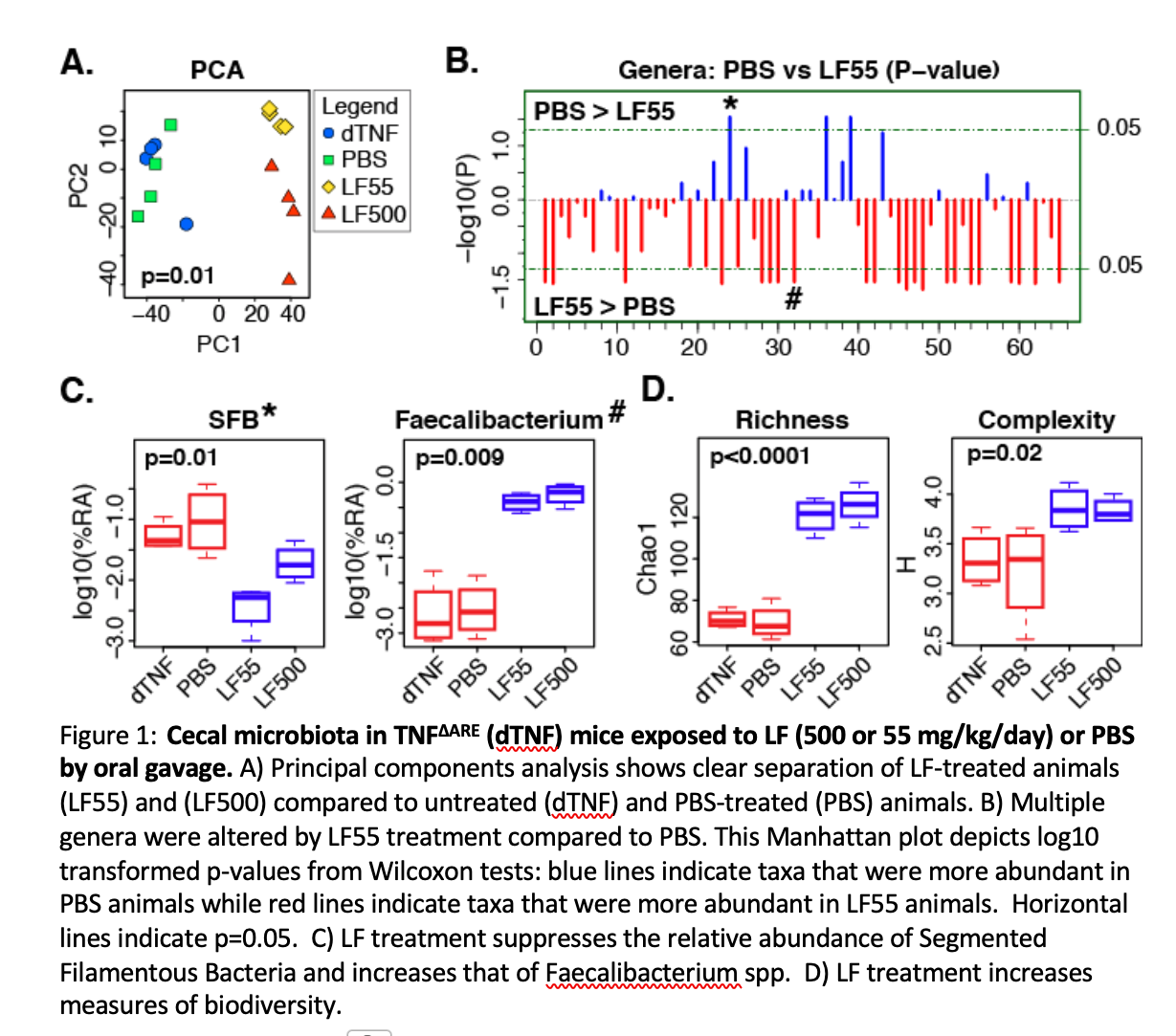
Results: Treatment of TNFΔAREmice with orally administered LF decreased ileitis with a significant decrease in Th17 inflammatory cells (12.3%+/- .7% vs 8.2%+/- 1.2%) and a concominant increase in anti-inflammatory regulatory T cells (18.9% +/- 1.3% vs 28.1 +/- 3.2%). Upon evaluation of the cecal bacteria of treated mice we noted that lactoferrin (rhLF55 or rhLF500) altered both the composition (Figure 1A, B, C) and diversity (Figure 1D) of the cecal microbiota. The alterations in the microbiome correlated well with the changes in immune cell predominance and the increase in the the anti-inflammatory genus Faecalibacteriumand Treg-skewing SCFA-producing Firmicutes, along with decreased levels of Th17-skewing Segmented Filamentous Bacteria (SFB) (Figure 1C).
Conclusion: The above data demonstrates that rhLF treatment results in a shift of the balance of pro- and anti-inflammatory T cell populations to favor resolution of gut inflammation and identifies a plausible mechanism for this shift.
.jpg )