Introduction: Acute rejection (AR) is a major complication in intestinal transplantation (ITX) that can lead to graft loss. Tacrolimus (TAC) is a mainstay of immunosuppression following ITX to prevent AR. Drug monitoring is routinely employed to ensure adequate exposure to TAC, while limiting adverse effects. Despite established practice, there is limited direct evidence linking therapeutic TAC levels with improved clinical outcomes post-ITX.
Method: This was a single-center review of all adult non-liver containing ITX graft recipients from 2010-2017, who maintained a functioning graft for 1 month. Patients received anti-thymocyte globulin (ATG) induction, with plasmapheresis and IVIG for positive T-cell CDC cross matches (CXM). Maintenance immunosuppression included TAC and prednisone, with mycophenolate or sirolimus added at physician discretion. 12-hour TAC whole blood trough levels were monitored daily in the hospital and every 1-4 weeks following discharge, with target levels of 13-17ng/mL month 1, 12-15ng/mL months 2-3, 10-13ng/mL months 3-6, and 8-12ng/mL months 6-12 post-ITX. Percent time-in-therapeutic range (TTR) for TAC was calculated from the date of transplant until 1 year post-ITX or graft failure using Rosendaal’s method (Rosendaal.Thromb Haemost.1993;69:236-239.) and patients were divided into TTR quartiles. Multivariable regression was used to identify variables associated with the incidence of AR on biopsy tissue within 1 year of ITX. Additional variables assessed included: graft type, adjunctive immunosuppression, age, sex, ethnicity, indication for ITX, prior transplants, cold ischemia time, DSA > 2,000 MFI, and positive CXM.
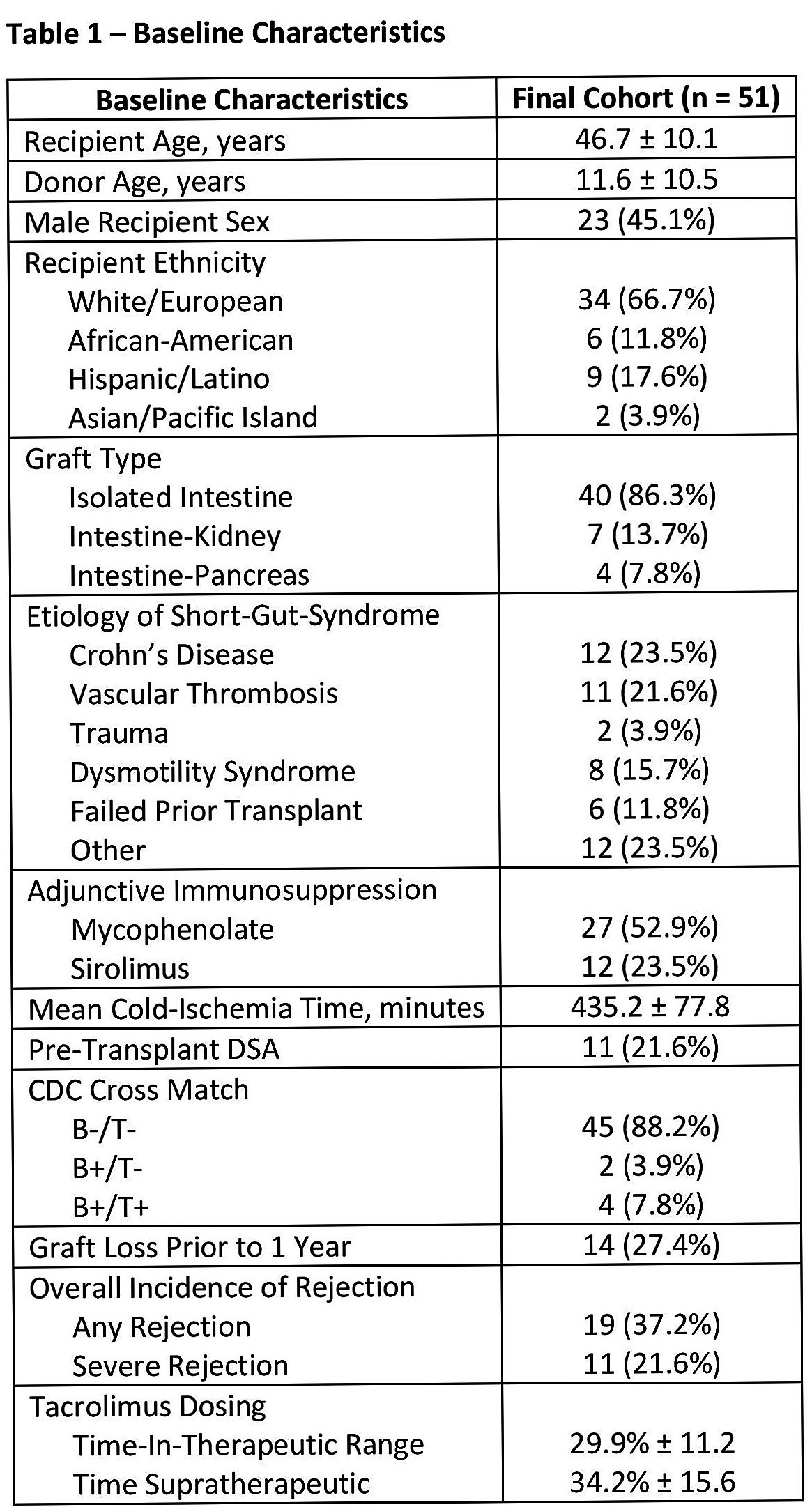
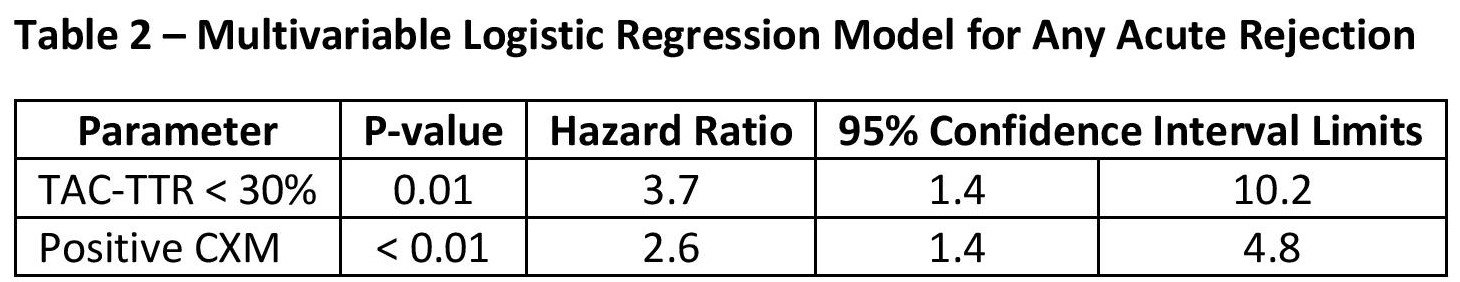
Results: A total of 51 patients were included in the analysis, 11 of which had pre-transplant DSA, and 6 patients had a positive CXM at time of ITX (Table 1). Mean TAC-TTR for the cohort was 29.9% ± 11.2, and 19 episodes of AR were observed in the first year post-ITX. Logistic regression demonstrated TAC-TTR<30% was associated with an increasing risk for any rejection episode (Table 2). A similar trend were observed for severe rejection episodes requiring ATG, with TAC-TTR<20% associated with a greater risk for severe rejection (p=0.02;HR:7.7[95% CI:0.9,65.3]).
Conclusion: Our dataset suggests that decreasing TAC-TTR may be a risk factor for both the occurrence and severity of rejection. If confirmed, our results indicate a need for utmost vigilance in TAC trough monitoring within the first-year post-ITX.
.jpg )